Dersler
-

Combining the Gas Laws ( Dr. Bülent BELİBAĞLI )
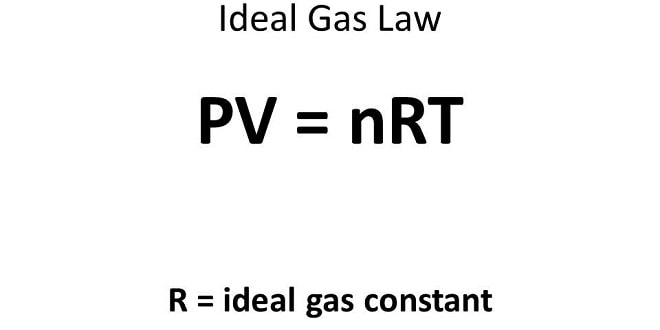
Combining the Gas Laws: (The Ideal Gas Equation and the General Gas Equation) The ideal gas equation is a single equation that includes…
-

Examples ( Dr. Bülent BELİBAĞLI )
Example: What are the partial pressures of H 2 and He gases in the gaseous mixture described in the previous example ?…
-

Thermochemistry ( Dr. Bülent BELİBAĞLI )
The part of the universe we choose to study is called a system. The parts of the universe with which the system…
-

Examples ( Dr. Bülent BELİBAĞLI )
Example: What quantity of heat is associated with the complete combustion of 1 kg of sucrose ? Solution: …
-

Boiling and the Boiling Point ( Dr. Bülent BELİBAĞLI )
Boiling and the Boiling Point When a liquid is heated in a container open to atmosphere, vapor bubbles form within the bulk of the liquid,…
-

Critical Point and Supercritical Fluids ( Dr. Bülent BELİBAĞLI )
Critical Point and Supercritical Fluids (SCF) If a liquid is heated in a sealed container, boiling doesn’t occur. The temperature and vapor pressure…
-

Solutions and Their Physical Properties ( Dr. Bülent BELİBAĞLI )
Solutions are homogeneous mixture of 2 or more substances (Chp 4). – Uniform throughout. • Solvent. – Determines the state of matter…
-

Ionic Solutions ( Dr. Bülent BELİBAĞLI )
Ionic Solutions e.g., NaCl in H2O 1. Ions that are less tightly held because of their position at a corner or an…