Genel Kimya
-

Average Atomic Mass ( Dr. Bülent BELİBAĞLI )
Average Atomic Mass The atomic mass (weight) of an element is the average of the atomic masses, weighted according to the naturally occuring abundances of the isotopes of the element. Example: What is the atomic weight of Mg ? The element consists of 78.70 % atoms (mass = 23.99 u), 10.13 % atoms (mass = 24.99 u) and 11.17 % atoms (mass = 25.98 u). …
-

Chemical Compounds ( Dr. Bülent BELİBAĞLI )
Types of Chemical Compounds and Their Formulas The combination of symbols, known as chemical formula, is used to represent a compound. The chemical formula of compound indicates; 1) the elements present 2) the relative numbers of atoms of each element in the compound. A formula unit is the smallest collection of atoms on which the formula of a compound can be based. A molecule of a compound is a group of bonded atoms that actually exist and can be identified as a distinct entity. Ionic Compuounds Atoms of almost all…
-

Establishing Empirical and Molecular Formulas ( Dr. Bülent BELİBAĞLI )
Establishing Empirical and Molecular Formulas 08.10.2013 Consider the following 5 step approach to determine formulas: 1. Choose an arbitrary sample size (100 g). 2. Convert masses of the elements in 100 g sample to amounts in moles. 3. Write a tentative formula based on the #s of moles just determined. 4. Divide each of the subscripts by the smallest one. 5. If all subscripts differ only slightly from whole #s, then, round them off. Otherwise multiply all subscripts by a small whole number to make the subscripts integral. …
-

Chemical Reactions ( Dr. Bülent BELİBAĞLI )
Chemical Reactions and the Chemical Equation A chemical reaction is a process in which one set of substances called reactants is converted to a new set of substances called products. As reactants are converted to products we observe: – Color change – Precipitate formation – Gas evolution – Heat absorption or evolution Chemical Reaction Step 1: Write the reaction using chemical symbols. Step 2: Balance the chemical equation. In a balanced equation the total number of atoms of each element is the same on both sides. …
-

Solution Dilution ( Dr. Bülent BELİBAĞLI )
Solution Dilution 22.10.2013 In general, it is not practical to store solutions of every possible concentration. Instead, we generally store fairly concentrated solutions, so-called stock solutions. Dilute solutions, then, can be prepared from stock solutions. A concentrated solution has a relatively large amount of dissolved solute. A dilute solution has a small amount. When a solution diluted, the amount of solute remains constant between the initial (i) and final (f) solution prepared. …
-

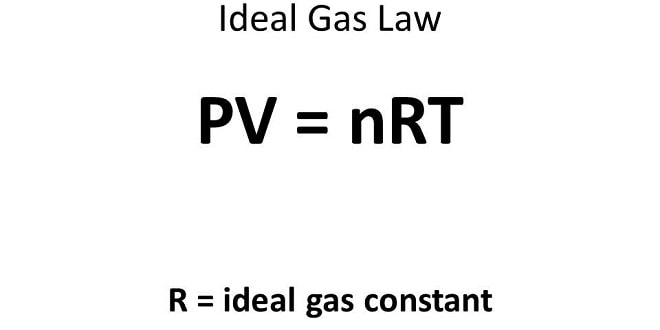
Combining the Gas Laws ( Dr. Bülent BELİBAĞLI )
Combining the Gas Laws: (The Ideal Gas Equation and the General Gas Equation) The ideal gas equation is a single equation that includes all four gas variables; V, T, P, amount of gas (n). Any gas that obeys this equation is said to be an ideal gas (or perfect gas). Example: What is the volume occupied by 13.7 g Cl2 at 45⁰C and 745 mmHg ? Solution: Example: How many molecules of N2 remained in an ultrahigh vacuum system of 128 mL volume when the pressure is reduced to 5×10-10 mmHg at 25⁰C ?…
-

Examples ( Dr. Bülent BELİBAĞLI )
Example: What are the partial pressures of H 2 and He gases in the gaseous mixture described in the previous example ? Solution: Total pressure of wet gas is equal to atmospheric pressure (P bar ) if the water level is the same inside and outside. Example: In the following reaction 81.2 mL of O 2 (g) is collected over water at 23⁰C and barometric pressure 751 mmHg. What must have been the mass of Ag 2 O(s) decomposed ? (Vapor pressure of H 2 O at 23⁰C = 21.1 …